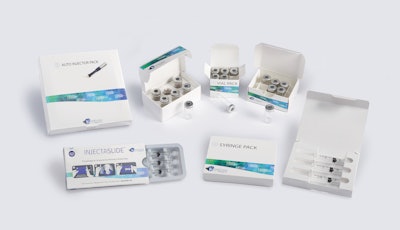
Keystone Folding Box Co., a designer and cGMP manufacturer of paperboard packaging, has introduced a line of secondary packaging systems for injectable pharma products. Available in a variety of tailored features and in both all-paperboard and plastic-hybrid formats, these options are available for prefilled syringes, auto-injector pens, vials and ampoules, as well as combinations of these.
Biologics, biosimilars, vaccines and other medications delivered through injection present unique secondary packaging challenges. Addressing these complexities, Keystone’s collection meets a range of needs through options such as tamper-evidence and child-resistance via reclosable locking mechanisms—all while ensuring product protection throughout the supply chain. The portfolio includes:
• The Vial Pack line of cartons includes designs for packaging a single vial or ampule, or as many products as a program requires.
• A line of Injectable Packs is suitable for prefilled syringes or auto-injector pens, and can be designed to include ancillary items like sterile wipes.
• The InjectaSlide package incorporates a thermoformed tray that slides inside a carton and into a locked position; a lock-release button must be pressed to unlock and slide the tray forward for easy access to each dose.
Keystone’s secondary packaging solutions for injectables are appropriate for automated or manual production, and are suitable for both clinical trials and full-scale commercialization. Materials used are compatible with the low-temperature exposure typically found in cold chain distribution of injectable products.
Child-resistant features are available with several of the options, and, for in-home use, multi-dose packs can be designed capable of prompting patients to administer the correct dose at the proper date and time.






















