LiNA Medical is one of the first medical device manufacturers to use the durability and high-flow processing advantages of Tritan™ copolyester from Eastman Chemical Co. LiNA employs the material in the medical device market with the introduction of the MaxFlow and FleXeal 5™ devices. Both products were featured at Eastman booth at the Medical Design & Manufacturing West trade show Feb. 8 to 10 in Anaheim, CA.
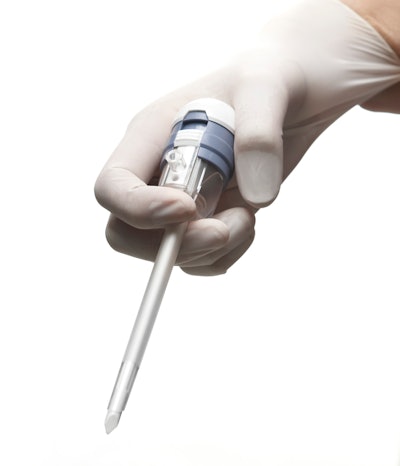
The MaxFlow Suction and Irrigation system single-use device for minimally invasive gynecological procedures features an ergonomic handle and color-coded push buttons with marked suction and irrigation labels. The FleXeal 5 is a 5-mm trocar system designed to complement laparoscopic surgical procedures.
LiNA had previously manufactured the MaxFlow with a different copolyester material, but by utilizing Tritan in existing molds, it reportedly decreased injection and processing time of the device housing by approximately 60%. Additionally, the toughness, heat and chemical resistance of Tritan allow the MaxFlow and FleXeal 5 to meet accelerated-aging protocols and withstand ethylene oxide (EtO) sterilization.
“Material specification for medical device development has many requirements, as products must meet regulatory demands and pass biocompatibility testing, sterilization, and accelerated-aging protocols,” said Henrik Bisgaard Poulsen, business unit manager, minimally invasive gynecology, LiNA Medical. “Switching to produce the MaxFlow with Eastman Tritan™ copolyester MX731 allowed LiNA Medical to create a device that has clarity without black specks and heat and chemical resistance for sterilization.
“Developing some of the first medical devices made of Eastman Tritan™ copolyester has helped LiNA Medical be an industry leader in proactively meeting marketplace demands,” Poulsen said. “As we continue to look for ways to improve our products, we anticipate working with Eastman and Tritan in the future.”
The MaxFlow Suction and Irrigation system single-use device for minimally invasive gynecological procedures features an ergonomic handle and color-coded push buttons with marked suction and irrigation labels. The FleXeal 5 is a 5-mm trocar system designed to complement laparoscopic surgical procedures.
LiNA had previously manufactured the MaxFlow with a different copolyester material, but by utilizing Tritan in existing molds, it reportedly decreased injection and processing time of the device housing by approximately 60%. Additionally, the toughness, heat and chemical resistance of Tritan allow the MaxFlow and FleXeal 5 to meet accelerated-aging protocols and withstand ethylene oxide (EtO) sterilization.
“Material specification for medical device development has many requirements, as products must meet regulatory demands and pass biocompatibility testing, sterilization, and accelerated-aging protocols,” said Henrik Bisgaard Poulsen, business unit manager, minimally invasive gynecology, LiNA Medical. “Switching to produce the MaxFlow with Eastman Tritan™ copolyester MX731 allowed LiNA Medical to create a device that has clarity without black specks and heat and chemical resistance for sterilization.
“Developing some of the first medical devices made of Eastman Tritan™ copolyester has helped LiNA Medical be an industry leader in proactively meeting marketplace demands,” Poulsen said. “As we continue to look for ways to improve our products, we anticipate working with Eastman and Tritan in the future.”





















