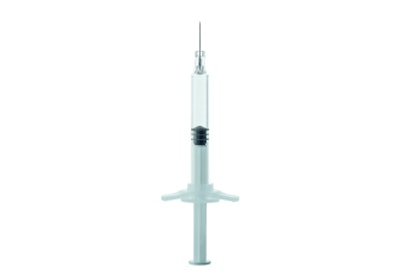
Pharmaceuticals and health care specialist Gerresheimer has commenced with series production of the new Gx RTF ClearJect syringes. The products of the high-performance plastic COP are used for sophisticated medication packaging. The new Gx RTF ClearJect syringe 1-mL long with cannula is the first plastic syringe developed and produced by Gerresheimer.
Gerresheimer brought together its competence in glass production at the Bünde location and the expertise of its plastic specialist in the Technical Competence Center (TCC) Wackersdorf for the development and industrialization of the new syringe. The production facility was planned and built by TCC’s automation team; the know-how for the quality control and quality guidelines for primary packaging originates from the inspection specialists of the glass location Bünde, and was adapted to the specific requirements of plastic production.
Production takes place to the high-quality requirements for pharmaceutical primary packaging material corresponding to GMP. The new production facility’s entire processing chain from injection molding to finished RTF packaging is covered. A series of cameras were installed at various stations for quality control, with which all relevant geometrical parameters and cosmetic-visual defects are checked for each individual product.
Innovative medications mean strict requirements for their primary packaging, especially biotechnologically manufactured active agents that may not interact with the packaging because this could mean undesirable effects for patients. Gx RTF ClearJect syringes offer low interaction potential.
COP has a high pH tolerance, does not emit metal ions into the medication solution, and therefore causes no displacement of the pH value while in storage. The syringe is manufactured as an injection-molding part in an eight-fold hot runner mold. The cannula is injected at the same time and does not need to be retroactively glued in. Tungsten and glue residue is therefore eliminated with the Gx RTF ClearJect syringes. The precise geometry of the injection-molding part reduces the dead volume in the syringe, leaving behind less of the expensive medication in the syringe. Also attractive is the increased safety for the end consumer. COP is particularly break-resistant, making it suitable for packaging aggressive or toxic materials.
The new Gerresheimer Gx RTF ClearJect COP syringe is available in the long 1-mL size with cannula. The design corresponds to ISO 11040-6. The first version of the syringe is equipped with a 27-gauge, 1/2-in. (12.7 mm), and thin-walled stainless-steel needle with three bevels.
The syringes are siliconized with a precisely controlled amount of high-viscosity, low-particle Dow Corning 360 MD (12,500 cSt) silicone oil, offering a comprehensive syringe system that completes the COP syringe body with plunger rods, plunger stoppers, finger flange, and closure systems. An economically efficient complete solution is achieved through the use of commercially available standard components.