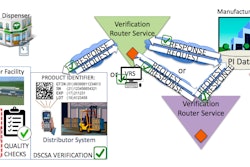
Last week, the FDA released two new guidances for interpreting track and trace requirements:
Data and documentation
The guidance document on data and documentation elaborates on the standards for the interoperable exchange of transactioninformation (TI), transaction history (TH), and transaction statements (TS) required by section 582 of the Federal Food, Drug, and Cosmetic Act. The document also:
-
Addresses how product tracing requirements apply to certain prescription drugs that entered the pharmaceutical distribution supply chain before January 1, 2015.
-
Aims to help trading partners understand which data elements should be included in the product tracing information, particularly in situations where trading partners are permitted by law to provide other trading partners with product tracing information that omits certain elements that would otherwise be required.
-
Recommends documentation practices that trading partners can use to satisfy the product tracing requirements of section 582. This guidance does not address all provisions of the DSCSA.
“As FDA works to implement other provisions of the DSCSA, the Agency expects to issue additional guidance and/or regulations and conduct public meetings to further delineate the requirements of the DSCSA.
Definitions of suspect and illegitimate product
The other document—brief in length—defines suspect product and illegitimate product as they appear in section 581 of the FD&C Act, also addressing the terms “diverted,” “counterfeit,” “fraudulent transaction” and “unfit for distribution.”
The agency notes that it is issuing this guidance in order to assist trading partners in meeting verification obligations, and help industry identify suspect and illegitimate product in the prescription drug distribution system in U.S.