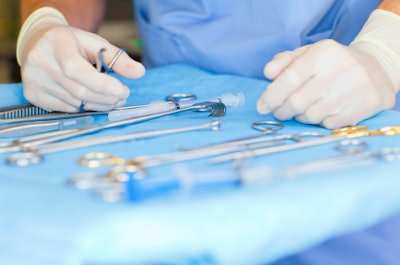
It's the holiday season, and as 2012 quickly approaches, the last thing any of us might want to consider is the risk of infection from inadequate reprocessing of reusable surgical and diagnostic medical devices. The U.S. Food and Drug Administration, however, is targeting this risk.
In an article posted to its Web site Dec. 28, the agency noted the following: "FDA has received reports of patients being exposed to microscopic amounts of blood, body fluids and tissue from other patients that may have occurred because the reusable devices were inadequately reprocessed and these contaminants were not removed. Transmission of infection was extremely rare, but the potential for becoming infected by an inadequately processed device was there."
The article said, "FDA is working with manufacturers and healthcare providers to:
• Make sure that the makers of these devices are providing reprocessing instructions that are clear and scientifically validated.
• Make sure that staff at hospitals and other healthcare facilities understand and are following the manufacturers' instructions.
• Identify device designs that facilitate optimal cleaning, disinfecting and sterilization.
And FDA has created a new Web site with information about these medical tools. To report a problem, the site also provides a link to MedWatch, the FDA Safety Information and Adverse Event Reporting Program."
Redesigned Web site
Meanwhile, the agency recently refreshed its Web site in an ongoing effort to make it easier for consumers, health professionals, industry, and the scientific community to access information. Changing images/stories give the site more of a consumer feel. For example, recent stories on the home page focused on acetaminophen dosing for infants, a new blog that on Dec. 27 had Jeffrey Shuren "Answering the Device Industry's Call for Clarity," and an article entitled, "Get Set for Winter Illness Season."
In an article posted to its Web site Dec. 28, the agency noted the following: "FDA has received reports of patients being exposed to microscopic amounts of blood, body fluids and tissue from other patients that may have occurred because the reusable devices were inadequately reprocessed and these contaminants were not removed. Transmission of infection was extremely rare, but the potential for becoming infected by an inadequately processed device was there."
The article said, "FDA is working with manufacturers and healthcare providers to:
• Make sure that the makers of these devices are providing reprocessing instructions that are clear and scientifically validated.
• Make sure that staff at hospitals and other healthcare facilities understand and are following the manufacturers' instructions.
• Identify device designs that facilitate optimal cleaning, disinfecting and sterilization.
And FDA has created a new Web site with information about these medical tools. To report a problem, the site also provides a link to MedWatch, the FDA Safety Information and Adverse Event Reporting Program."
Redesigned Web site
Meanwhile, the agency recently refreshed its Web site in an ongoing effort to make it easier for consumers, health professionals, industry, and the scientific community to access information. Changing images/stories give the site more of a consumer feel. For example, recent stories on the home page focused on acetaminophen dosing for infants, a new blog that on Dec. 27 had Jeffrey Shuren "Answering the Device Industry's Call for Clarity," and an article entitled, "Get Set for Winter Illness Season."