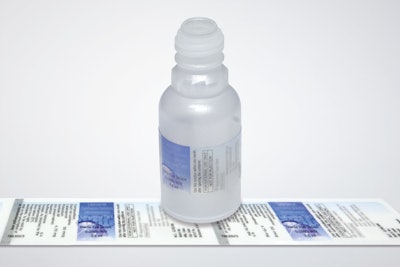
Schreiner MediPharm has developed customer-specific, low-migration labels for plastic containers that do not compromise the medicines contained within. As a result, special adhesive systems, materials, and inks are qualified to develop the ideal label design for each particular plastic container.
Increasingly, pharmaceutical manufacturers are using primary containers made of plastics; however, soft plastic materials have a drawback: Molecules from solvents and other substances contained in inks, adhesives, and film materials could migrate through the packaging over the years. For example, eye drops in soft plastic bottles might be contaminated or their effectiveness impaired.
To ensure that safe materials are used for each particular label design, Schreiner MediPharm conducted a study with an independent test institute to analyze, calculate, and evaluate the migration tendency of various label compositions. The results made it possible to specifically develop low-migration labels for each application.





















