Technology equipment provider Siemens has teamed up with Adents, a provider of easily deployable serialization and track-and-trace software solutions, to develop a combined hardware and software solution providing drug manufacturers with item-level serialization capabilities at a competitive cost.
The new joint offering comprises Siemens’ range of powerful and reliable standard equipment—including industrial PCs, software controllers, I/Os and camera barcode readers—with Adents’ Pharma Suite software, which manages all serialization parameters from a centralized server at site level (Level 3) with fast implementation and minimal personnel involvement.
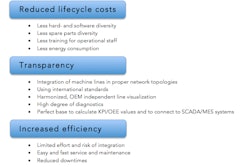
Equipment from Siemens is well regarded for its reliability, ruggedness and ease of maintenance, while Adents’ serialization software enjoys a reputation for flexibility and cost-effectiveness. The companies’ combined solution can easily be integrated into existing infrastructure and equipment, and has the capacity to handle both existing and future regulatory requirements. This means that the unit-level track-and-trace rules can be modified continuously for a wide variety of production types.
Other benefits of the new Siemens-Adents solution include:
• Fewer qualification tests needed and exceptional performance on high-speed packaging lines, each helping to maximize line productivity
• Solution and implementation management that meet the pharmaceutical industry’s highest quality standards, such as the GAMP5 guidelines
• The support of a global network of certified Siemens Solution Partners for quick and simplified integration
• Support and service delivered globally by Siemens
New laws and regulations pertaining to drug traceability are already in place or pending enactment with the goal of preventing drug counterfeiting worldwide. Over the next few years, more than 70% of prescription drugs on sale worldwide will need to be identifiable at unit level. This mandates most pharmaceutical manufacturers in about 40 countries, including the U.S. and European Union, to implement unit-level serialization and aggregation processes on their packaging lines.
Complying with these new regulations entails major changes to production facilities, information systems, quality control and the structure of the industry's supply chain. This transformation represents a major industrial challenge in the years to come for the pharmaceutical industry.
“The issues are multiple and complex—budget control, risk of loss of productivity, variety of industrial equipment and implementation planning. Drug manufacturers will therefore have to be very careful when it comes to choosing their serialization solution. The combined expertise of Siemens and Adents will provide drug manufacturers with the best item-level serialization solution, with a competitive cost,” says Vincent Masztalerz, Head of the Business Unit Process Automation in France.
“The collaboration between Siemens and Adents sets up a new deal in the track-and-trace global market. Combining a fully configurable software suite, a complete range of standard equipment and an extensive network of global integration partners represents the most efficient and future-proof approach to serialization and aggregation in the pharma industry,” adds Christophe Devins, CEO and Founding Partner of Adents. “It will enable pharmaceutical companies and contract manufacturers to easily and quickly address the current regulatory requirements and remain in compliance long term.”