This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
AptarGroup, Inc., a global leader in drug and consumer product dosing, dispensing and protection technologies, announced that it was awarded a contract from the U.S. Federal Government to advance development of its ActivShield™ technology. This innovative solution sterilizes medical devices and instruments without the need for a power source, making it a versatile solution for numerous environments, including rural areas, military settings and healthcare facilities with limited or no current sterilization capability.
ActivShield™ technology leverages Aptar CSP Technologies’ 20+ years of material science expertise and is built upon the company’s proven 3-Phase Activ-Polymer™ platform, a highly engineered active material science solution trusted by global brands to protect sensitive drug products, medical devices, drug delivery systems, and probiotics.
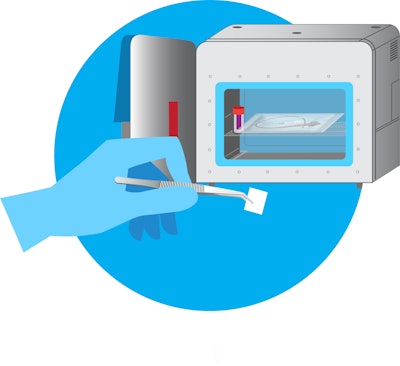
ActivShield™ technology is a portable, novel sterilization modality that does not require conventional infrastructure, power, or extensive training, and more importantly, does not present the health risk associated with the use of Ethelyne Oxide (EtO). ActivShield™ uses a highly engineered Activ-Film™ material that emits a controlled amount of chlorine dioxide gas to sterilize a wide range of medical devices and instruments.
“As an innovation leader in pharma dosing, dispensing and protection technologies, we are proud to work with the U.S. Government to advance our ActivShield™ solution for the sterilization of medical devices and instruments in locations where a power source may not be available, further enhancing its versatility,” said Stephan B. Tanda, Aptar President and CEO.
Aptar’s technology can be custom engineered to meet the needs of specific devices and conditions. Its stability throughout shelf life and compact size (Figure 1) makes it easy to stockpile, and all materials are manufactured in the United States.
ActivShield™ technology is well-positioned to fill unmet sterilization needs in remote or emergency response civilian settings, which could significantly reduce infection risks stemming from inadequately sterilized medical devices and instruments. Additionally, the technology could reduce reliance on expensive and unsafe sterilization techniques such as EtO, which has come under recent scrutiny by the EPA for toxic emissions.
For the military, ActivShield™ can help wounded service members, particularly those in far-forward environments, have access to sterilized instruments during critical pre-hospitalization periods, following severe injuries.
John Belfance, president of Aptar CSP Technologies, stated, “ActivShield™ technology is a breakthrough in material science technology that has the potential to dramatically expand reliable instrument sterilization for challenging environments, simplifying processes and helping to save lives. We are grateful for the government’s recognition, which supports the potential of this new technology, and look forward to bringing ActivShield™ technology to the forefront of sterilization techniques.”
The five-year contract is valued at approximately $4.8 million.
* This work is supported by the US Army Medical Research and Development Command under Contract No. HT9425-24-C-0078. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.






















