Exciting medical treatment advances in the years ahead may well stem from the creative deployment of combination products (combinations of biologics, medical devices, and/or pharmaceuticals). Associating combination products with the future is understandable, yet it might surprise some to learn that many companies producing these ever-developing products have been at it for more than 20 years.
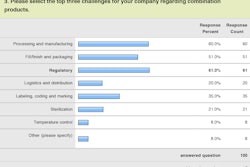
That was one of the findings of an exclusive survey conducted in late 2013 by Healthcare Packaging. Exactly 100 manufacturer/end-user professionals answered the e-mail survey. Package development/engineering/R&D represented the largest respondent group. Because most of the questions permitted more than a single answer, the actual response count to those questions surpassed 100, as evidenced in the accompanying charts.
When we asked what precise bit of information would best help your company succeed in its combination product packaging efforts, regulatory issues were mentioned often. Respondents sought guidance on matters ranging from “regulatory requirements for combination products that have multiple suppliers” to a “monthly overview of country-specific regulations and possible pathways to comply with those regulations.” Other requests included information on “validation, FDA regulations, and track and trace,” and “clearer regulatory pathway knowledge.”