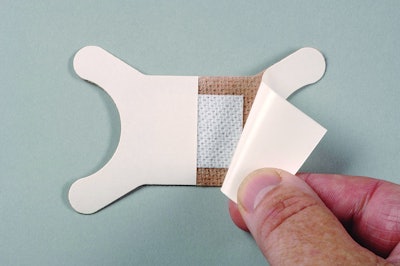
Fabrico Medical, a provider of converted materials and advanced assembly for the design-to-manufacture of medical devices and disposables, now offers low-trauma silicone adhesives from 3M™ for gentle skin interface.
Low-trauma soft silicones provide good adhesion to a variety of skin types, including the fragile skin conditions of the very old and very young. These adhesives cause low mechanical trauma during dressing, bandage re-positioning, and removal. They don’t stick to hair, don’t attract dry or dead skin cells, and leave the skin contact area intact, lowering chances of skin stripping, tearing, and infection.
Fabrico Medical works with design engineers on wound care dressings and bandages to identify the best silicone formulations for the application and also slit, laminate, die-cut, and package the finished product. With in-house materials and adhesives lab testing capabilities, Fabrico Medical can explore alternatives in the design stage, to enhance the design and ensure excellent design for manufacturability. Fabrico Medical works closely with 3M to provide the exact adhesive solution and dressing package required by the application.






















