By October 1, the European Medicines Agency (EMA) plans to start the next phase in its business continuity plan, safeguarding “core activities related to the evaluation and supervision of medicines” while it scales back other activities in the face of relocation and staff losses.
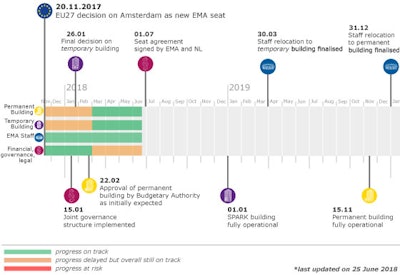
In Nov. 2017, the European Union Member States made the decision to relocate the EMA from the UK to Amsterdam. The agency is expecting significant staff loss of about 30%, with a “high degree of uncertainty regarding mid-term staff retention”.
As a recent EMA release explains, “The temporary cuts in activities are required because it has also become clear that the Agency will lose more staff than initially anticipated. Staff who will not relocate to Amsterdam have already started to leave the Agency and this trend is expected to accelerate. In addition, due to the employment rules in the Netherlands, 135 short-term contract staff will no longer be able to work for EMA.”
Next steps
While the staff losses are startling, the agency has plans for short and long-term progress outlined in the aforementioned business continuity plan. Phases 1 and 2 have been implemented, and phase 3 begins with scaling back some non-essential activities by October 1, including collaboration at the international level, which will be temporarily scaled back to focus on topics such as product-related requests and supply-chain integrity.
Development and revision of guidelines will be limited to those that address urgent public or animal health needs, or those related to Brexit.
Harmonization of global medicine regulations will also take a backseat temporarily, while the EMA notes that “engagement in other global public health issues such as antimicrobial resistance or vaccines will be maintained as long as possible, but reviewed on a case-by-case basis.”